Reactant-favored Product-favored Insufficient information 2 A g 3 B g 4C g ΔΗ -95 kJ 2 A g B s 3 C g AH -193 kJ A g B g 3 C g Question. The value of K_c Increases Decreases Remains the same The value of Q_c Is greater than K_c Is equal to K_c Is less than K_c The reaction.
13 7 The Gibbs Free Energy Chemistry Libretexts
Where possible classify these systems aS reactant-favored or product-favored at 298 K.
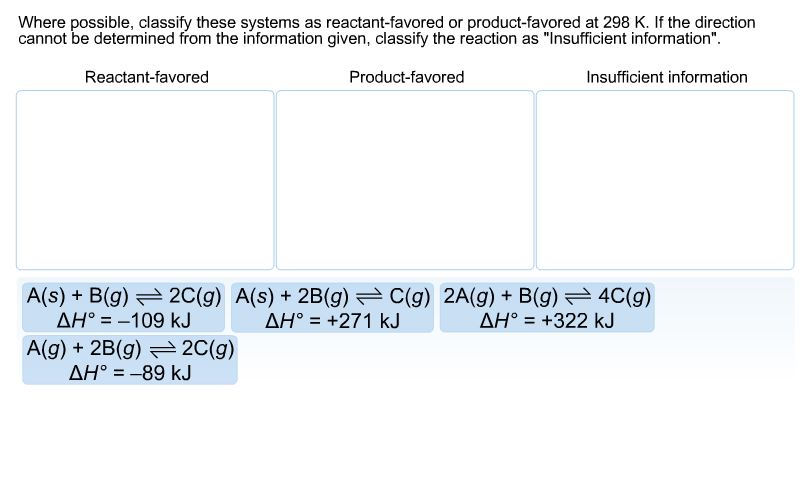
. Where possible classify these systems as reactant-favored or product-favored at 298 K. If the direction cannot be determined from the information given classify the reaction as Insufficient information. A2A g 3B g --4C g delta H -95kJ b2A g 2B g --3C g delta H.
Where possible classify these systems as reactant-favored or product-favored at 298 K. If the direction cannot be determined from the information given classify the reaction as Insufficient information Reactant-favored Product-favored Insufficient information Answer Bank A gB g 3 C g AH 388 kJ A g 2 B g 2 C g A sB g. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
Where possible classify these systems as reactant-favored or product-favored at 298 K. 23250 results page 23. The factor which is used to measure the spontaneity or favourable condition of the reaction is gibbs free energy and it will be calculated as.
Where possible classify these systems as reactant-favored or product-favored at 298 K298 K. Consider the reaction A B AB. If the direction cannot be determined from the information given classify the reaction as Insufficient information.
Which factor is used to measure the spontaneity. If the direction cannot be determined from the information given classify the reaction as Insufficient information_ Reactant-favored Product-favored Insufficient information 2 Ag 3 Bg 4 Cg AH 95 kJ 2 Alg Bs 3 Cg 4H -193 kJ Ag Bg 3 Cg AH 388 kJ 2 Ag Bg. Where possible classify these systems as reactant-favored or product-favored at 298 K.
At 700 K ΔG 34 kJ and ΔG -07 kJ. If the direction cannot be determined from the information given classify the reaction as Insufficient information Reactant-favored Product-favored Insufficient information AO SO V 2 A B 3 C AH -193 S AH 2B 2 5CC AH. Chemistry questions and answers.
If the direction cannot be determined. The reaction has an equilibrium constant of Kp226x104 at 298 K. Kc 17 10 3 0034 2x2 017 x01 x Now take the square root of both sides of this equation.
As Bg 2Cg delta H degree -109 kJ 2. Where possible classify these systems as reactant-favored or product-favored at 298 K. Where possible classify these systems as reactant-favored or page 23.
If the direction cannot be determined from the information given classify the reaction as Insufficient information. Where possible classify these systems as reactant-favored or product-favored at 298 K. If the direction cannot be determined rom the information given classify the reaction as Insufficient information.
A2A g 3B g --4C g delta H -95kJ. Where possible classify these systems as reactant-favored or product-favored at 298 K. Where possible classify these systems as reactant-favored or product-favored at 298 K.
If the direction cannot be determined from the information given classify the reaction as Insufficient information A. Answered expert verified. Up to 256 cash back Consider the following system at equilibrium where Delta H degree -161 kJ and K_c 154 at 298 K.
What is favored at equilibrium at 700 K and which direction does the reaction proceed to reach equilibrium. If the direction cannot be determined from the information given classify the reaction as Insufficient information. ΔG ΔH - TΔS.
But at room temperature which is 298K it will be spontaneous and proceed left to right. 2 NO g Br_2 g 2 NOBr g If the TEMPERATURE on the equilibrium system is suddenly decreased. The forward reaction is favored.
If the direction cannot be determined from the information given classify the reaction as Insufficient information Reactant-favored Product-favored Insufficient information 2Ag 2Bg 근 5Cg 2Ag 3Bg-4Cg 2Ag 2Bg 근 3Cg H -95 kJ 2Ag Bs 근. This is the sublimation of iodine Use the reaction I2s. Hence it is reactant favored reaction.
Where possible classify these systems as reactant-favored or product-favored at 298 K. Where possible classify these systems as reactant-favored or product-favored at 298 K. B2A g 2B g --3C g delta H 254kJ.
Science Chemistry QA Library Vhere possible classify these systems as reactant-favored or product-favored at 298 K. COg 2H_2g CH_3OHg Calculate Kp for the reactions and predict whether reactants or products will be. Forward reaction favored concentration of products is higher Which direction of the reaction equilibrium is favored at 298 K room temperature use the reaction I2 solid to I2 gas when H 624 kJmol.
The direction of the reaction is favored at 298 kelvin is in the backward direction as gibbs free energy is positive. If the direction cannot be determined from the information given classify the reaction as Insufficient information. Reactants are favored and the reaction proceeds in the forward direction to reach equilibrium.
Where possible classify these systems as reactant-favored or product-favored at 298 K. C2A g B g -- 4C g delta H 322 kJ.
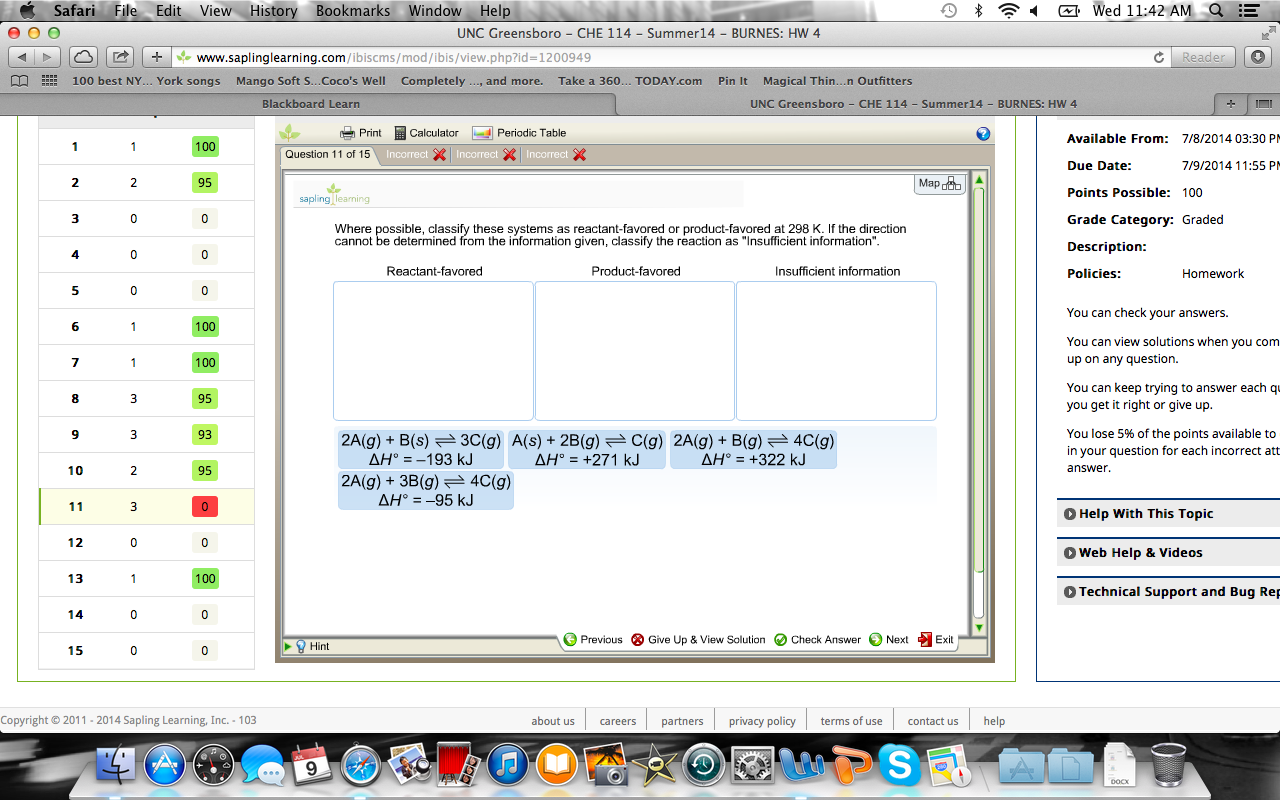
Solved Where Possible Classify These Systems As Chegg Com

15 6 The Reaction Quotient Predicting The Direction Of Change Chemistry Libretexts

Solved Where Possible Classify These Systems As Chegg Com

Thermodynamics Pt 2 Dr Harris Lecture 22 Ch 23 11 19 12 Ppt Download
0 Comments