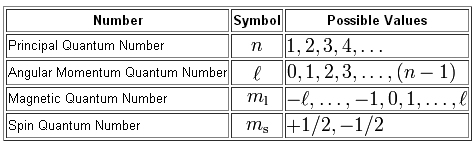
Identify the sets of quantum numbers that describe all the electrons in the ground state of a neutral beryllium atom Be. L has both numbers and letters associated with it.

Quantum Number Orbital Definition Formula Diagram Shape
N 4l 2ml 2ms 1 2.
. Drag the appropriate items to their respective bins. The spin quantum number ms specifies the electron spin. The principal quantum number n identifies the shell in which the electron is found.
Identify the sets of quantum numbers that describe all the electrons in the ground state of a neutral beryllium atom Be. Quantum Numbers Principal Azimuthal Magnetic and Spin - The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. L0s l1p l2d l3f In a 3p orbital n3 since its the first number and l1 since when l1p.
Each set is ordered nℓmℓms. The magnetic quantum number me distinguishes the orbitals within a subshell. Drag the appropriate items to their respective bins.
Up to 256 cash back The principal quantum number n identifies the shell in which the electrorn is found. N ℓ mℓ and ms. N2 l3 ml1 N is any integer.
Each set is ordered in l me m. To learn Detailed Explanation of Different Types of Quantum Numbers Visit BYJUS for more. The angular momentum quantum number indicates the kind of subshell.
N3 l0 ml1 E. N 4l 2ml 1ms 1 2. Chemistry questions and answers.
You will thus have 10 sets of quantum numbers that can be used to describe an electron located in one of the five d-orbitals. N 4l 2ml 1ms 1 2. N3 l2 ml1 C.
The angular momentum quantum number ℓ indicates the kind of subshell. There are four quantum numbers namely principal azimuthal magnetic and spin quantum numbers. N3 l1 ml1 D.
Every electron in an atom is described by a unique set of four quantum numbers. N1 l3 ml1 B. Drag the appropriate items to their respective bins.
21012210-12100-12200-122001221-1-121001221-112 1 Electrons in Be. Chemistry questions and answers. The magnetic quantum number mℓ distinguishes the orbitals within a subshell.
N 4l 2ml 2ms 1 2. View Available Hint s Reset Help 21012 210-12 21-1-12 10012 200-12. Which of the following sets of numbers can describe a 3p electron.
Identify the sets of quantum numbers that describe all the electrons in the ground state of a neutral beryllium atom Be. Each set is ordered n l me m. View Available Hint s Reset Help 21-112 21012 10012 21-1-12 200-12 100-12 210-12 20012 Electrons in Be.
Ml 2 or ml 1 or ml 0 or ml 1 or ml 2.

Quantum Numbers Introduction To Chemistry

How To Find The Quantum Numbers Of An Element Study Chemistry With Us Youtube

Light And The Modern Atom Teaching Chemistry Chemistry Education Physics And Mathematics
0 Comments